Chemical Sensitivity
Changing the local chemical environment (e.g., atomic bonding) has little effect on the x-ray and Auger deexcitation processes. However, electron energy loss spectroscopy (EELS), x-ray photoelectron spectroscopy (XPS), and cathodoluminescence (CL) can elucidate local chemical changes.
EELS – As the core electron is promoted to empty states just above the Fermi level, it strongly couples with the local density of (unoccupied) states (LDOS). The LDOS directly relates to the electrons bonding in the material. In the orbital hybridization picture of bonding, these are the anti-bonding states. This is analogous to x-ray absorption spectroscopy (XAS) typically performed at synchrotron beamlines.
XPS – The ejected photoelectron energy relates to the difference between the core level of the photoelectron and the vacuum level. Bonding and coordination will cause a chemical shift of this energy. By comparing the unknown spectra to standards, you can determine the fraction of each chemical state present.
Fingerprinting – Recreating the EELS or XAS fine structure from first principles is not straightforward. Several research programs focus on making these first principle calculations more robust, but many body and quantum effects make the calculations challenging. An alternative approach is to compare the measured spectrum to a data library to gain insight into the material you study.
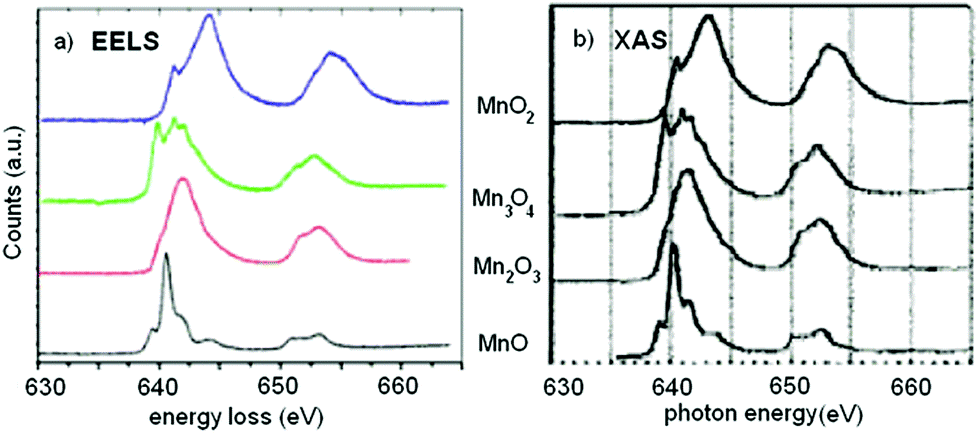
Goode, A. E.; Porter, A. E.; Ryana, M. P.; McComb, D. W. Nanoscale, 2015, 7, 1534 – 1548.